PI Name & Affiliation:
Dr. R. Rajasekaran,
Associate Professor
School of Bio Sciences and Technology (SBST)
Vellore Institute of Technology, India
Co-PI Name & Affiliations:
Dr. George Priya Doss,
Associate Professor
School of Bio Sciences and Technology (SBST)
Vellore Institute of Technology, India
Funding Agency: ICMR
Scheme: Ad hoc
Overlay: Rs. 14,83,000
Duration of the Project: 2 Years

Dr. R. Rajasekaran

Dr. George Priya Doss
Project Description
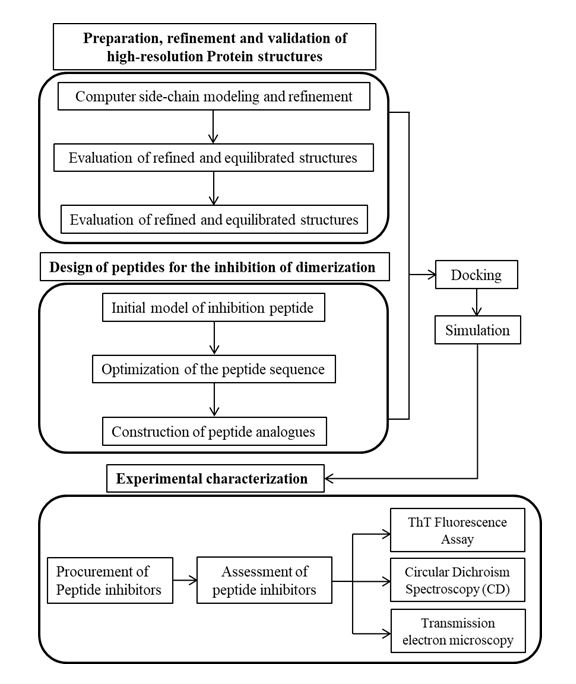
Parkinson’s disease (PD) is characterized by the selective loss of dopaminergic neurons in substantia nigra region of brain and the formation of intracellular Lewy bodies and Lewy neuritis. Research findings stipulated that α-synuclein is a vital component of filaments in Lewy bodies and Lewy neuritis of PD. Moreover, the accrual of α-synuclein in Lewy bodies and neurites in the form of amyloid fibrils are considered to be the key features of pathogenicity in PD. Hence, impeding the formation of eccentric amyloid fibril of α-synuclein, thereby preventing the neuronal death could be a possible strategy for the treatment of PD. Although, α-synuclein(αS) has been the subject of experimental studies, atomic-level structural information regarding the aggregate formation that is sufficient for the rationale design of αS inhibitors are not available. Such structures are very difficult to obtain by the experimental methods alone because of the loss in the structural stability and intermediate folding of the protein. In the proposed research, molecular simulation tools will be used to carry out the rationale in silico design of αS inhibitor peptides by solving the aggregated structure of αS using modelling algorithm and further, validating the effect of the inhibitor peptide on the aggregated protein by resolving the complex structures with the recent development in the physics-based computer modelling algorithm and experimental characterization.