PI Name & Affiliation:
Dr. G. Thirumanavelan,
Associate Professor
School of Advanced Sciences (SAS)
Vellore Institute of Technology, India
Funding Agency: DST-SERB
Scheme: Core Research Grant (CRG)
Overlay: Rs. 34,06,315
Duration of the Project: 3 Years

Dr. G. Thirumanavelan
Project Description
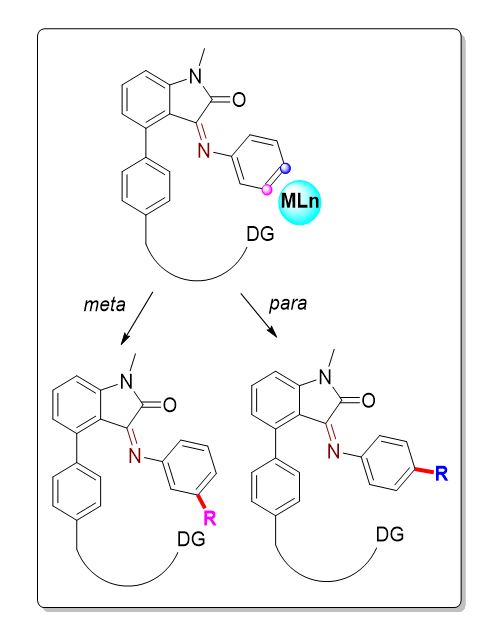
Aromatic hydrocarbons are a very common framework that occurs in natural products, pharmaceuticals, agrochemicals, polymers, and functional materials. The activation and functionalization of a specific C-H bond become a major challenge owing to the presence of multiple C-H bonds. Strategies like imposing sterics, inherent reactivity, or directing group to the concerned substrate engage in a discriminative way to effect the site- and stereoselective C-H activation/functionalization. Focusing on directing group methodology, often, energetically favourable five- or six-membered cyclometallation was exploited in organic transformations which yield ortho-substituted aromatics. However, functionalizing the sp2 C-H at distal positions, i.e., meta or para is challenging.
To target these remote sites, substrates were attached with the following moieties: removable templates - acting as directing groups, quaternary salts - via ion-pair directing, and Lewis-base site - effects the Lewis-pair generated sterics. Understandably, employing a ligand which is commercial and economic significance (isatin) that possesses a carbonyl functionality could act as a transient directing group. The carbonyl functionality in isatin reacts with aniline substrates to form imines which in turn governs the spatial and geometry of the reaction site to activate the remote sp2 C-H functionalization.